Henry's law of adsorption. Langmuir adsorption isotherm equation. Lyophilic disperse systems. Classification and general characteristics of pav. Thermodynamics and mechanism of micellization. The structure of surfactant micelles in aqueous and hydrocarbon media. Solubilization
In the case of the interaction of two atoms:
U is the interaction energy;
U = U + U PULL.
 - Lennard-Jones equation
, c, b, m = const
- Lennard-Jones equation
, c, b, m = const
In cases of interaction of atoms with a solid surface, it is necessary to sum up all interactions.
x is the distance to the surface
r - the radius of action of the forces of attraction
dV - volume
n is the number of surface molecules
U ADS. is the adsorption interaction energy



In the case of adsorption, attraction is enhanced. And in the case of interaction of the nonpolar-nonpolar type, adsorption is predominantly localized in the depressions.
electrostatic interaction.
Polar adsorbent - non-polar adsorbate
Non-polar adsorbent - polar adsorbate
A polar adsorbent is a polar adsorbate.
M  the adsorbate molecule is represented as a dipole, and the adsorbent as a conductor, in which the adsorbate molecule induces a dipole mirror-symmetrically with respect to the given one.
the adsorbate molecule is represented as a dipole, and the adsorbent as a conductor, in which the adsorbate molecule induces a dipole mirror-symmetrically with respect to the given one.
X - distance to the middle
When interacting, the potential arises:
 ,
,
 is the dipole moment.
is the dipole moment.
The potential tends to take on the maximum value, i.e. dipoles tend to orient themselves perpendicular to the surface.
Since an increase in temperature promotes the growth of Brownian motion, it leads to a deceleration of the adsorption process.
In the case of electrostatic interaction, the adsorbate is predominantly localized on protrusions.
Fundamental adsorption equation.
In the case of adsorption, the component is redistributed, which means that the chemical potential changes. The process of adsorption can be considered as the transition of surface energy into chemical energy.
Layer volume = 0, then the generalized equation I and II of the law of thermodynamics:
T = const; (1) = (2) => 

For a two-component system:
,
 ,
,
 =>
=>
=>
 - Gibbs adsorption equation
.
- Gibbs adsorption equation
.
For the case of adsorption of TV. body - gas:, 
 ,
,

 - isotherm
- isotherm
 - isobar
- isobar
 - isopykne
- isopykne
 - isostere
- isostere
Isotherm, isopycne, isostere are related to each other.
Because adsorption function 



Henry isotherm Langmuir isotherm



Thermodynamics. Adsorption.
For condensed media:
 ,
,
 ,
,
 - integral change in the Gibbs energy
.
- integral change in the Gibbs energy
.

P-pressure over a curved surface, P S-pressure over a flat surface
 - adsorption potential
- adsorption potential
Differential change in entrapy 
 , Г = const
, Г = const
- differential entropy change
- differential enthalpy of adsorption
 - isosteric heat of adsorption
- isosteric heat of adsorption
 - heat of condensation
- heat of condensation
 - net heat of adsorption
- net heat of adsorption

 ,
,




Qa is the integral heat of adsorption, 
Qra is the integral net heat of adsorption,

Henry's equation
The study of adsorption is hampered by the inhomogeneity of the surface, so the simplest regularities are obtained for homogeneous surfaces.
Let us consider the interaction of gases with a solid surface, when a gas passes from an equilibrium state in the volume to an equilibrium state on the surface. This case is analogous to the equilibrium of gases in a gravitational field.
 ,
,
 ,
=>
,
=> -Henry's equation
-Henry's equation
 - distribution coefficient
- distribution coefficient
In the process of adsorption, a change in chemical potentials occurs.
For bulk phase: 
For surface gas: 
In a state of equilibrium  , i.e.
, i.e.

In the Henry equation, the constant does not depend on the concentration
The Henry equation is valid in the region of low pressures and concentrations. As the concentration increases, 2 types of deviations from Henry's law are possible:
1 - positive deviations, D decreases, A decreases
2 - negative deviations, D - increases, A - increases.
The type of deviation is determined by the predominance of one or another type of adsorbent-adsorbate interaction.
With a strong adhesive interaction, the activity coefficients increase - a positive deviation. In the case of cohesive interactions, negative deviations are observed.
monomolecular adsorption.
Langmuir isotherm.
The simplest regularities were obtained in Henry's theory. Langmuir proposed a theory according to which adsorption is considered as a quasi-chemical reaction. Wherein:
The surface is energetically uniform.
Adsorption is localized, each adsorption center interacts with one adsorbate molecule.
Adsorbate molecules do not interact with each other.
Adsorption is monolayer.

 - surface,
- surface,  - adsorbate,
- adsorbate,  - adsorption complex.
- adsorption complex.
 , then the concentration of adsorption sites:
, then the concentration of adsorption sites:  ,
, - limiting adsorption.
- limiting adsorption.
 , then the reaction constant:
, then the reaction constant: 

 - Langmuir equation.
- Langmuir equation.
Adsorption versus Concentration
1 )
)
 ,
,
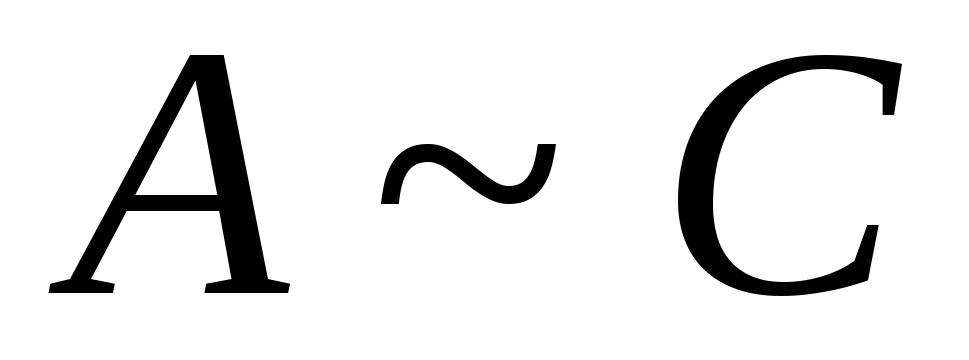
2) area of high concentrations
 - limiting adsorption, formation of a monomolecular layer
- limiting adsorption, formation of a monomolecular layer
For the Gibbs energy: .
g is the entropy factor.
In the case of the Henry isotherm, the Gibbs energy characterizes the transition of the adsorbate from the standard state in the bulk to the standard state on the surface. In the case of the Langmuir isotherm  characterizes the degree of affinity of the adsorbent and adsorbate.
characterizes the degree of affinity of the adsorbent and adsorbate.
 found from the van't Hoff isobar.
found from the van't Hoff isobar.

 , then
, then  , hence
, hence  .
.

 - degree of surface filling.
- degree of surface filling.




 - the number of vacancies,
- the number of vacancies,  - the number of occupied places.
- the number of occupied places.
 ,
,

Those. in the region of high concentrations, the number of free sites is inversely proportional to the amount of adsorbate.
Adsorption of a mixture of gases on a homogeneous surface.
In this case, the adsorption process is considered as two parallel reactions.
 (1)
(1)

 (2)
(2)


Adsorption of a mixture of gases on an inhomogeneous surface.
In the case of a non-homogeneous surface, one should not limit oneself to medium infills.
As a result of competition, localization of various adsorbates is possible in different types of sites.
In this case, the relation  .
.
 ,
,
 is the saturation vapor pressure of the adsorbate.
is the saturation vapor pressure of the adsorbate.
 ,
,
 is the heat of adsorption.
is the heat of adsorption.
"+" - symbatic dependence, "-" - antibatic dependence, "H" - no correlation.
"+" - adsorption proceeds according to the same mechanism. In the most energetically favorable areas, a gas with a high affinity to the surface is predominantly adsorbed.
"-" - adsorption proceeds through various mechanisms and up to a certain point in time there is no competition for the surface.
Monomolecular adsorption is predominantly realized during the physical adsorption of gases at low values p, as well as at the liquid/gas interface.
Polymolecular adsorption.
BET theory(Brunauer, Emmet, Teller).
In the case where the formation of a monolayer is insufficient to compensate for the surface energy, adsorption is polymolecular and can be considered as a result of forced condensation under the action of surface forces.
Basic provisions:
When an adsorbate molecule hits the occupied site, a multiple set is formed.
As you get closer p to p s the number of free adsorption sites decreases. Initially, the number of places occupied by singles, doubles, etc. increases and then decreases. kits.
At p =p s adsorption turns into condensation.
There are no horizontal interactions.
For the first layer, the Langmuir isotherm is performed.
The surface is considered as a set of adsorption sites. The condition of dynamic equilibrium is valid: the rate of condensation in free places is equal to the rate of evaporation from occupied ones.
a is the condensation coefficient (the fraction of molecules condensed on the surface);
 ,
,
Zm is the maximum number of free places.
 - frequency of vibrations of atoms in the direction perpendicular to the surface.
- frequency of vibrations of atoms in the direction perpendicular to the surface.
For the first layer, the dynamic equilibrium conditions are:



 , then
, then
 - Langmuir equation.
- Langmuir equation.
For the second layer will be true: 
For the i-th layer: 
For simplicity, it is assumed that a and ν are the same for all layers except the first one. For all layers except the first, the heat of adsorption is constant. For the last layer, the heat of adsorption is equal to the heat of condensation. As a result, the equation
 (*)
(*)
C- constant,
In the case of BET theory, the constant FROM characterizes the Gibbs energy of pure adsorption. The equation contains only one constant, and this equation is also very important for determining the specific surface area of the adsorbent.
Since heat is released as a result of adsorption, the determination of specific surfaces is carried out at low temperatures.
 ????????????
????????????


The main flaw of the theory– Neglect of horizontal interactions in favor of vertical ones.
The equation is in the range  from 0.05 to 0.3.
from 0.05 to 0.3.
Where  <
0,05 – существенное влияние оказывает
неоднородность поверхности.
<
0,05 – существенное влияние оказывает
неоднородность поверхности.
 > 0.3 - the interaction of the adsorbate - adsorbate affects.
> 0.3 - the interaction of the adsorbate - adsorbate affects.
Accounting for adsorbate-adsorbate interactions.
Interactions appear during adsorption on a nonpolar surface of branched molecules or molecules. capable of forming associations. In this case, the shape of the adsorption isotherms changes.
BUT  the sorbent is not polar.
the sorbent is not polar.
Graph 1 corresponds to weak interactions adsorbate-adsorbate, strong adsorbate-adsorbent.
Graph 2 corresponds to a strong adsorbate-adsorbate interaction, a strong adsorbate-adsorbent interaction.
Graph 3 corresponds to a strong adsorbate-adsorbate interaction, a weak adsorbate-adsorbent interaction.
 ,
,

In the case of interaction between adsorbate molecules, it is necessary to take into account changes in the activity coefficients. And this equation is written as:
 - the equation of Frunkin, Fowler, Guggenheim.
- the equation of Frunkin, Fowler, Guggenheim.
k is the attraction constant.
Polan's potential theory.
This theory does not derive any type of adsorption isotherm, but makes it possible to calculate isotherms at a different temperature.
Adsorption is the result of the attraction of the adsorbate to the surface of the adsorbent due to the action of the adsorption potential, which does not depend on the presence of other molecules and depends on the distance between the surface and the adsorbate molecule.
 ,
,
 - adsorption potential.
- adsorption potential.
Since the surface is inhomogeneous, the distance is replaced by the adsorption volume  .adsorption volume is the volume enclosed between the surface and the point corresponding to the given value
.adsorption volume is the volume enclosed between the surface and the point corresponding to the given value  .
.
Adsorption potential is the work of transferring 1 mol of the adsorbate outside the given adsorption volume to a given point of the adsorption volume (or the work of transferring 1 mol of saturated vapor of the adsorbate, which is in equilibrium with the liquid adsorbate in the absence of the adsorbent, into the vapor phase in equilibrium with the adsorbent).

Characteristic curve
 - adsorption potential,
- adsorption potential, 
For a given adsorbent and various adsorbates, the following is true: 
For different types of adsorbates  ,
,
where  potentials for adsorption isotherms at relative pressures
potentials for adsorption isotherms at relative pressures  for adsorbate 1 and for adsorbate 2. This ratio is a constant value.
for adsorbate 1 and for adsorbate 2. This ratio is a constant value.
 - affinity coefficient
- affinity coefficient
Theory of capillary condensation.
The course of the adsorption process largely depends on the structure of the porous body.
|
microporous | |
|
Transitional porous | |
|
Macroporous |
In the case of microporous sorbents, the fields of adsorption forces overlap. In the case of macroporous sorbents, the pores act as transport channels. The processes of condensation are most significant in transient porous bodies. Capillary condensation starts at certain values p And  when part of the surface energy has already been compensated. A necessary condition is that the surface must be self-supporting. The process is described Thompson-Kelvin equation.
when part of the surface energy has already been compensated. A necessary condition is that the surface must be self-supporting. The process is described Thompson-Kelvin equation.

 - for the case of wetting, the center of curvature is in the gas phase.
- for the case of wetting, the center of curvature is in the gas phase.
In the case of capillary condensation, the adsorption isotherm has a hysteresis form. The lower branch corresponds to the adsorption process, and the upper branch corresponds to the desorption process.
All types of pores can be reduced to three types:
|
conical |
Cylindrical with one closed end |
Cylindrical with two open ends |
|
Process filling is carried out from the bottom of the pore. The adsorption isotherm and the desorption isotherm in this case coincide, since the adsorption process begins with a sphere and the desorption process also begins with the disappearance of some spheres.
↓ |
There is no hysteresis. Forward and reverse stroke are described by the equation:
|
There is no bottom anywhere, the filling of the pore will go along the walls of the cylinder.
cylinder: Isotherm and will have a hysteresis form.
↓ |
IN  wetting conditions, condensation occurs at lower pressures, which is energetically favorable. From the desorption branch, pore size distribution curves are obtained.
wetting conditions, condensation occurs at lower pressures, which is energetically favorable. From the desorption branch, pore size distribution curves are obtained.
The maximum of the differential curve is shifted to the left relative to the inflection point of the integral. The total volume of small pores is small, but has large surface areas. As the pore size increases, their volume increases as  , and the area as
, and the area as  , due to this, a shift of the maximum of the differential curve is observed.
, due to this, a shift of the maximum of the differential curve is observed.
Adsorption at the solid-liquid interface.
In the case of adsorption at the solid-gas interface, we neglected one component. In the case of adsorption at the solid-liquid interface, the adsorbate displaces solvent molecules from the surface of the adsorbent.
 ,
,
The right equation is:

 ,
,
N 1, N 2 - mole fractions of the solvent and component, N 1 + N 2 \u003d 1, then
 ,
=>
,
=>
 , then - the adsorption equation for the phase boundary solid - liquid.
, then - the adsorption equation for the phase boundary solid - liquid.
Adsorption (G) > 0 at  <
0
<
0
If the values  for the component and solvent are very different, in this case the dependence G from N has an extremum at the value N
~ 0,5.
for the component and solvent are very different, in this case the dependence G from N has an extremum at the value N
~ 0,5.
E  if
if  have similar values, in this case the sign of adsorption may change. Addiction G from N crosses the x-axis
have similar values, in this case the sign of adsorption may change. Addiction G from N crosses the x-axis
Function Intersection G(N) with the abscissa axis is called adsorption azeotrope. This means that the two components cannot be separated on this adsorbent.
Adsorption isotherm equation with exchange constant.
During adsorption at the solid-liquid interface, the components are constantly redistributed between the surface of the adsorbent and the volume of the solution.
 - components (- - refer to the surface)
- components (- - refer to the surface)

 ,
,
 ,
, .
.
 ,
,


Adsorption at the liquid-gas interface
R  Let us consider the change in the concentration profile as the liquid-gas interface is crossed. Let component 2 be volatile.
Let us consider the change in the concentration profile as the liquid-gas interface is crossed. Let component 2 be volatile.
Cs is the concentration in the surface layer.
Based on the definition of excess adsorption

If the component is not volatile, then the adsorption value will be written as follows:
P  ri
ri 

In the equation  the nature of matter is described by the derivative
the nature of matter is described by the derivative  .
.
The surface tension isotherm can be of the form 1 or 2:
1 - surfactants
2 - surfactants
Surface activity g is the ability of substances to reduce surface tension in the system.

 - thickness of the surface layer
- thickness of the surface layer
C s is the concentration of the component in the surface layer
FROM– volume concentration
For a homologous series there is a rule:
 - Traubeau Duclos rule
- Traubeau Duclos rule
For the homologous series, the adsorption isotherm looks like this:
We write D instead of A, since adsorption is excessive in the surface layer.
Surface Tension Isotherm:

 is the surface tension of the pure solvent.
is the surface tension of the pure solvent.
 - fundamental adsorption equation;
- fundamental adsorption equation;
 - Langmuir equation.
- Langmuir equation.
Let's solve them together:

- Shishkovsky's equation.
B is a constant for the homologous series.
A- when moving from one homologue to another, it increases by 3-3.5 times 
![]()
1 - area of low concentrations
![]()
2 - average concentration
3 - monomolecular layer
Surfactants are amphiphilic molecules, i.e. include a polar group and a non-polar hydrocarbon radical.
o is the polar part of the molecule.
| is the non-polar part of the molecule.
In a polar solvent, surfactant molecules are oriented in such a way that the polar part of the molecule faces the solvent, while the nonpolar part is pushed into the gas phase.
In the Shishkovsky equation  , it is constant for the homologous series.
, it is constant for the homologous series.
Surface-active action begins to appear with n>5. At concentrations higher than the concentration of the monomolecular layer, micellization occurs in surfactant solutions.
Micelle- the aggregate of amphiphilic surfactant molecules is called, the hydrocarbon radicals of which form the core, and the polar groups are turned into the aqueous phase.
Micelle mass - micellar mass.
H  the number of molecules is the number of aggregation.
the number of molecules is the number of aggregation.
Spherical micelles
In the case of micellization, an equilibrium is established in the solution
CMC is the critical micelle concentration.
Since we consider the micelle to be a separate phase:
For the homological series, there is an empirical equation:
a is the dissolution energy of the functional group.
b is the adsorption potential increment, the work of adsorption per methylene unit.
is the adsorption potential increment, the work of adsorption per methylene unit.
The presence of a hydrocarbon core in micelles makes it possible for compounds that are insoluble in water to dissolve in aqueous solutions of surfactants, this phenomenon is called solubilization (what dissolves is a solubilizate, surfactant is a solubilizer).
The mud may be completely non-polar, may contain both polar and non-polar parts, and will be oriented like a surfactant molecule.
In any case, during solubilization, an increase in the micellar mass and aggregation number occurs not only due to the inclusion of the solubilizate, but also due to an increase in the number of surfactant molecules necessary to maintain the equilibrium state.
Solubilization is the more effective, the lower the molecular weight of the solubilizate.

 ~ 72 mN/m.
~ 72 mN/m.
 ~ 33 mN/m.
~ 33 mN/m.
The effectiveness of surfactants depends on the magnitude of the CMC.
2D Surface Layer Pressure

→ -forces of surface tension.
- two-dimensional pressure.
The surface layer is a force equal to the difference between the surface tensions of a surfactant solution and a pure solvent, directed towards a clean surface.
An equilibrium is established between the solution and the surface layer



At  there is an area where
there is an area where  linearly dependent on concentration.
linearly dependent on concentration.
G [mol / m 2].
 area occupied by one mole of a substance
area occupied by one mole of a substance
Then the two-dimensional pressure isotherm will have the form 
 is the two-dimensional pressure isotherm.
is the two-dimensional pressure isotherm.
Addiction  from S M:
from S M:

At  - two-dimensional pressure increases sharply. At
- two-dimensional pressure increases sharply. At  two-dimensional is deformed, which causes a sharp growth
two-dimensional is deformed, which causes a sharp growth  .
.
The film on both sides limited by the same phases is called double-sided. In such films, a constant movement of the mother liquor is observed.
Films less than 5 nm thick are called black films.

Adsorption layers should have two characteristics: viscosity and easy mobility, fluidity and elasticity.
The Marangoni effect is self-healing.
Gibbs Triangle,  - overpressure.
- overpressure.
The film is stretched and due to the fact that part of the liquid is gone, the surfactants rush into the free space. Gibbs triangle.
Effect of adsorption strength of bodies.
There is always an adsorption layer on the film surface, for which , then
Langmuir equation:


 into two-dimensional pressure
into two-dimensional pressure
 - analogue of the Shishkovsky equation
- analogue of the Shishkovsky equation
electrokinetic phenomena. Double electric layer (DES).
Helemholtz model. Gouy-Chapman theory.
1808 Flight

U – shaped tube, immersed in it 2 electrodes. The law of communicating vessels is violated and there is a change in the liquid level in the tube - electrokinetic phenomena.
Kinetic phenomena:
electrophoresis
Electroosmosis
Flow (flow) potential
Sedimentation potential
1 and 2 arise when a potential difference is applied, 3 and 4 the punching and sedimentation of colloidal particles cause the appearance of a potential difference.
Electroosmosis is the movement of a dispersion medium relative to a stationary dispersed phase under the action of an electric current.
electrophoresis is the movement of particles of the dispersed phase relative to a stationary dispersion medium under the action of an electric current.
P  The reason for the occurrence of electrokinetic phenomena is the spatial separation of charges and the appearance of a double electric layer.
The reason for the occurrence of electrokinetic phenomena is the spatial separation of charges and the appearance of a double electric layer.
The electric double layer is a flat capacitor, one plate is formed by potential-determining ions, the other by counterinoes. The ions are also contaminated as potential-determining co-ions are pushed into the bulk of the solution. Distance between plates  . The potential falls linearly, the potential difference
. The potential falls linearly, the potential difference  .
.
An external potential difference causes the appearance of a shear modulus  is a pair of forces per unit area acting along the surface of a solid body.
is a pair of forces per unit area acting along the surface of a solid body.

At equilibrium, the shear modulus is equal to the viscous friction modulus (  ).
).

In our conditions  ,
,

 - Helemholtz-Smalukovsky equation
- Helemholtz-Smalukovsky equation
 - linear speed displacement i phases.
- linear speed displacement i phases.
E is the electric field strength.
 - potential difference between the plates
- potential difference between the plates
 - electrophoretic mobility [m 2 /(V * s)].
- electrophoretic mobility [m 2 /(V * s)].
The Helemholtz model does not take into account the thermal motion of molecules. In reality, the distribution of ions in the double layer is more complex.
Gouy and Chapman identified the following causes of DES:
The transition of an ion from one phase to another when equilibrium is established.
Ionization of solid phase matter.
Completion of the surface by ions present in the dispersion medium.
Polarization from an external current source.
The electrical double layer has a blurred or diffuse structure. Ions tend to be evenly distributed throughout the diffuse layer.
The diffuse layer consists of counterinions, the length of the layer is determined by their kinetic energy. At a temperature tending to absolute zero, counterinoes are as close as possible to potential-determining ions.
This theory is based on two equations:
Boltzmann equation

 - work against the forces of electrostatic interaction.
- work against the forces of electrostatic interaction.
 is the bulk charge density.
is the bulk charge density.

Poisson equation 
Since the DEL thickness is much smaller than the particle size, and for a flat DEL, the derivative with respect to coordinates  And
And  is abolished.
is abolished. 
For e y with y<<1 функцию можно разложить в ряд Маклорена:

We restrict ourselves to two members of the series, then:


 - DEL thickness is the distance at which the DEL potential decreases in e once.
- DEL thickness is the distance at which the DEL potential decreases in e once.
The lower the temperature, the less  . At Т→0 – flat DES. The higher the concentration, the more I, the less
. At Т→0 – flat DES. The higher the concentration, the more I, the less  .
.





 “–” means that the potential decreases with distance. =>
“–” means that the potential decreases with distance. =>
=>

 ,
,
 - the potential decreases exponentially.
- the potential decreases exponentially.
Potential for surface charge density: 
Surface charge is a space charge with the opposite sign, integrated over distance.




=>


Where the potential decreases by 2.7 times - 
Double layer capacity 
The disadvantage of the theory is that the presence of the Helemholtz layer is not taken into account, i.e. does not take into account  , hence the errors in determining the main parameters. It also does not explain the effect of ions of different nature on the thickness of the electrical double layer.
, hence the errors in determining the main parameters. It also does not explain the effect of ions of different nature on the thickness of the electrical double layer.
Stern's theory. The structure of a colloidal micelle.
The electrical double layer consists of two parts: dense and diffuse. A dense layer is formed as a result of the interaction of potential-forming ions with specifically adsorbed ones. These ions, as a rule, are partially or completely dehydrated and can have either the same or the opposite charge to the potential-determining ions. It depends on the ratio of the energy of electrostatic interaction  and specific adsorption potential
and specific adsorption potential  . The ions of the dense layer are fixed. The other part of the ions is located in the diffuse layer; these ions are free and can move deep into the solution, i.e. from an area of higher concentration to an area of lower concentration. The total charge density consists of two parts.
. The ions of the dense layer are fixed. The other part of the ions is located in the diffuse layer; these ions are free and can move deep into the solution, i.e. from an area of higher concentration to an area of lower concentration. The total charge density consists of two parts.

 - Helmholtz layer charge
- Helmholtz layer charge
 -Diffuse layer charge
-Diffuse layer charge
The surface has a certain number of adsorption centers, each of which interacts with one counterion. The constant of such a quasi-chemical reaction is:

 , where
, where  - mole fraction of counterions in solution
- mole fraction of counterions in solution
Helmholtz distribution
The potential decreases linearly
Gouy Potential Distribution. There is no dense layer, the potential decreases exponentially from the value 
Stern distribution.
Initially, the potential decrease is linear, and then exponentially.
When an electric field is applied in the case of electrophoresis, it is not the particle of the solid phase that moves directly, but the particle of the solid phase with a layer of surrounding ions. DES repeats the shape of the particle of the dispersed phase. When a potential is applied, a part of the diffuse layer is torn off. The break line is called sliding boundary.
The potential arising at the slip boundary as a result of detachment of a part of the diffuse layer is called electrokinetic potential(Zeta potential  ).
).
A particle of a dispersed phase, with a layer of counterions surrounding it and a double electric layer, is called micelle.
Rules for writing colloidal micelles:


1-1 charging electrolyte
T is a particle of the dispersed phase.
AA is the boundary between the dense and diffuse parts.
BB is the slip boundary.
The slip boundary may or may not coincide with line AA.
The pH value at which the zeta potential is zero is called isoelectric point.
CaCl 2 + Na 2 SO 4 → CaSO 4 ↓ + 2NaCl
1. In excess of CaCl 2
CaCl 2 ↔ Ca 2+ + 2Cl -
(CaSO 4 m∙nCa 2+ 2( n-x)Cl - ) 2 x + x Cl - - record micelles.
CaSO 4 m - aggregate.
CaSO 4 m∙nCa 2+ is the core.
CaSO 4 m∙nCa 2+ 2( n-x)Cl - particle.
2. In excess of Na 2 SO 4
Na 2 SO 4 ↔2Na + + SO 4 2-
(CaSO 4 m∙nSO 4 2- 2(n-x)Na + ) 2x- 2xNa + - micelle
CaSO 4 m - aggregate.
CaSO 4 m∙nSO 4 2 + is the core.
CaSO 4 m∙nSO 4 2- 2(n-x)Na + - particle
Helemholtz-Smoluchowski equation

 - linear velocity of displacement of boundaries (in electroosmosis).
- linear velocity of displacement of boundaries (in electroosmosis).
 - potential difference on the capacitor plates (in electroosmosis).
- potential difference on the capacitor plates (in electroosmosis).


 - volumetric flow rate of the solution, S is the cross-sectional area of the cell.
- volumetric flow rate of the solution, S is the cross-sectional area of the cell.

E is the electric field strength.


 (for electroosmosis).
(for electroosmosis).
For the flow potential:

 - potential
- potential
 - membrane pressure
- membrane pressure
As a rule, the value of electrophoretic mobilities and electroosmotic mobilities is less than the calculated ones. This is due to:
Relaxation effect (during the movement of a particle of the dispersed phase, the symmetry of the ionic atmosphere is violated).
Electrophoretic braking (the occurrence of additional friction as a result of the movement of counterions).
Distortion of streamlines in the case of electrically conductive particles.
Relationship between surface tension and potential. Lippmann equation.
The formation of DEL occurs spontaneously due to the desire of the system to reduce its surface energy. In the context of constancy T And p the generalized equation of the first and second laws of thermodynamics looks like:
 (2)
(2)
(3), (1)=(3) =>
=>

 - 1st Lippmann equation.
- 1st Lippmann equation.
 is the surface charge density.
is the surface charge density.
 - differential capacitance.
- differential capacitance.
 - 2nd Lippmann equation.
- 2nd Lippmann equation.
FROM- capacity.
We solve the 1st Lippmann equation and the fundamental adsorption equation:
 ,
,


 , then
, then
 - Nernst equation
- Nernst equation
 ,
,
 ,
,




 - the equation of the electrocapillary curve (ECC).
- the equation of the electrocapillary curve (ECC).
IN  :
: , but
, but 
Cationic surfactants (CSAS) reduce the cathodic branch of the ECC.
Anionic surfactants (ASS) reduce the anodic branch of the ECC.
Non-ionic surfactants (NSA) reduce the middle part of the ECC.
Stability of disperse systems. Wedging pressure.
Dispersed systems can be divided:
Thermodynamically unstable systems can be kinetically stable due to the transition to a metastable state.
There are two types of stability:
Sedimentation stability (with respect to gravity).
Aggregative stability. (in relation to sticking)
Coagulation is the process of particles sticking together, leading to the loss of aggregative stability. Coagulation can be caused by changes in temperature, pH, stirring, ultrasound.
Distinguish coagulation:
Reversible.
Irreversible.
Coagulation proceeds with the introduction of electrolytes.
Coagulation rules:

Film- This is the part of the system located between two interfaces.
disjoining pressure occurs with a sharp decrease in the film thickness as a result of the interaction of approaching surface layers.

«-» - when the film thickness decreases, the disjoining pressure increases.
P 0 is the pressure in the bulk phase, which is a continuation of the interlayer.
P 1 is the pressure in the film.
Theory of stability. DLFO (Deryagin, Landau, Fairway, Overbeck).
According to the DLVO theory, two components are distinguished in the disjoining pressure:
electrostatic P E (positive, it is due to the forces of electrostatic repulsion). Corresponds to a decrease in the Gibbs energy with increasing film thickness.
Molecular PM (negative, due to the action of attractive forces). It is caused by the compression of the film due to chemical surface forces, the radius of action of the forces is tenths of a nm with an energy of the order of 400 kJ/mol.
Total interaction energy:

 - the system is aggregate stable
- the system is aggregate stable
 - unstable system
- unstable system
P  positive component.
positive component.
The increase is due to the increase in potential energy during compression of thin films. For thick films, the excess ion energy is compensated and is equal to the energy interaction in the bulk of the dispersion medium.
If  (
( - film thickness,
- film thickness,  - radius of the ion) thinning of the film leads to the disappearance and reduction in it of molecules and ions with a minimum surface energy. The number of neighboring particles decreases, as a result of which the potential energy of the particles remaining in the film increases.
- radius of the ion) thinning of the film leads to the disappearance and reduction in it of molecules and ions with a minimum surface energy. The number of neighboring particles decreases, as a result of which the potential energy of the particles remaining in the film increases.


The DLVO theory considers the interaction of particles as the interaction of plates.

Particles don't interact



 - Laplace equation,
- Laplace equation,  ,
,


For weakly charged surfaces
For highly charged surfaces:

The molecular component is the interaction of two atoms:
 ~
~
Interaction of an atom with a surface:

Let's take two records:
D  To obtain the molecular component, it is necessary to sum up all the interaction energies of the atoms of the right and left plates.
To obtain the molecular component, it is necessary to sum up all the interaction energies of the atoms of the right and left plates.
where  - Hamaker's constant (takes into account the nature of interacting bodies).
- Hamaker's constant (takes into account the nature of interacting bodies).
That. the interaction energy of particles in a system can be expressed using potential curves.
I is the primary potential minimum. This is a zone of irreversible coagulation, the forces of attraction prevail.
II - zone of aggregative stability, repulsive forces prevail.
III - secondary potential minimum (or flocculation zone). Between the particles of the dispersed phase there is an electrolyte layer, and the particles can be separated and transferred to the zone of aggregative stability.

Curve 1 – the system is aggregatively stable.
Curve 2 is stable in zone I, not stable in zone II.
Curve 3 - coagulation occurred in the system.
Curve 4 - at point 4, the total energy of interaction U=0,  , this extremum point corresponds to the onset of rapid coagulation.
, this extremum point corresponds to the onset of rapid coagulation.
There are two cases:
1. Surfaces are weakly charged:
U \u003d U E + U M \u003d 0
 (1)
(1)
2)

 (2)
(2)



 - this is the thickness of the layer corresponding to the beginning of the coagulation process.
- this is the thickness of the layer corresponding to the beginning of the coagulation process.






 - for weakly charged surfaces
- for weakly charged surfaces
 then
then 
2. For highly charged surfaces:
 (1)
(1)
2)

 (2)
(2)


 (3)
(3)
 ,
,
Let's square (3)

Coagulation:
In specific adsorption, ions can be adsorbed in a superequivalent amount in such a way that the surface can change its charge. The surface is being recharged.
In the case of specific adsorption, not only ions of opposite signs, but also of one ion can be adsorbed.
If ions of the same sign as the surface are adsorbed, then in the surface layer there will be not a drop in the potential, but its growth.

Neutralization coagulation (occurs with the participation of weakly charged particles and depends not only on the charge of the coagulating electrolyte, but also on the potential at the boundary of the dense and diffuse layers).
Smoluchowski's theory of rapid coagulation.

Dependence of coagulation rate on electrolyte concentration.
I – coagulation rate is low,
II - the coagulation rate is practically proportional to the electrolyte concentration.
III - the area of rapid coagulation, the rate is practically independent of concentration.
Key points:
The initial sol is monodisperse, similar particles have a spherical shape.
All particle collisions are effective.
When two primary particles collide, a secondary particle is formed. Secondary + primary = tertiary. Primary, secondary, tertiary - multiplicity.
In terms of chemical kinetics, the coagulation process can be described by the equation:

The solution will be the equation: 
 - time of half coagulation. This is the time during which the number of sol particles decreases by 2 times.
- time of half coagulation. This is the time during which the number of sol particles decreases by 2 times.

 ,
,
 ,
,
 ,
,


As the multiplicity increases, the maximum of the coagulation curves shifts towards larger values  .
.
Disadvantages:
Assumption of monodispersity.
The assumption about the effectiveness of all collisions.
Henry adsorption isotherm equation
If we consider the dynamic picture of adsorption, then its value will be the greater, the greater the number of impacts of gas molecules on the surface (i.e., the greater the gas pressure) and the longer the time the molecule stays on the surface from the moment of impact to the moment it passes back into the gas phase . Therefore, but de Boer, the adsorption value
where n is the average number of molecules hitting the surface per unit time, τ is the average residence time of the molecules on the surface. This formula assumes that each impact of a molecule is accompanied by its delay on the surface, regardless of whether there are already other molecules on it or not. In reality, a molecule hitting an already occupied place may be reflected back into the gas phase or delayed, but its retention time will be different.
Accounting for these circumstances led to the following formula:
This is the Henry adsorption isotherm equation. It means that in the ideal model the amount of adsorption is directly proportional to the vapor or gas pressure. This dependence received this name by analogy with Henry's law known in physical chemistry, according to which the volume of a gas dissolved in a solid or liquid is proportional to its pressure. So, according to the accepted assumptions, the Henry isotherm should describe the experimental data obtained at low fillings on homogeneous surfaces. The first assumption, as was said, is justified in the study of adsorption at very low pressures. As for the second, adsorption is almost always measured on inhomogeneous surfaces. However, adsorption at very low pressures corresponds to very low degrees of coverage. This means that everything depends on how inhomogeneous not the entire surface is, but only a small fraction of it, which is covered at low pressures. Therefore, in the literature one can find enough examples of both kinds. The constant K of the Henry equation (the tangent of the slope of the straight line) depends on the temperature and the interaction energy of the adsorbate - adsorbent, as can be seen from equation (4.4). The lower the temperature and the greater the interaction of adsorbed molecules with the surface of the adsorbent, the greater K, the steeper the adsorption isotherm.
Of course, the assumption that molecules are adsorbed with the same probability on any part of the surface, including those already occupied earlier, is too rough an assumption, suitable only for very small degrees of coverage. Another assumption can be made, which is that adsorption occurs only on free areas of the surface and that any hit of molecules on already occupied places does not lead to an adsorption event. This assumption is equivalent to the postulate of monolayer adsorption and, as we said earlier, it actually holds in the case of chemical adsorption, but the situation is more complicated for physical adsorption.
Another assumption made in the derivation of the Henry isotherm equation is that the surface is homogeneous, i.e. equivalence of all its sections, we will keep unchanged. And, finally, the third assumption in the new model under consideration is the absence of interaction between adsorbed molecules, i.e. we will assume that the residence time of a molecule on the surface does not depend on where it hits - in the immediate vicinity of another molecule or at a great distance from it. All these assumptions were made by Langmuir in the derivation of the adsorption isotherm, which he made in 1918.
The Langmuir adsorption isotherm equation can be derived in various ways. Langmuir himself derived it by considering the dependence of the rates of adsorption and desorption on the degree of surface coverage and assuming that at equilibrium both rates become the same.
The thermodynamic derivation of this equation was given by Volmer, and the statistical derivation by Fowler.
In this form, the Langmuir equation is widely known. It contains two constants: a m briefly called monolayer capacity (maximum adsorption), and K is a constant depending on adsorption energy and temperature.
adsorption isotherm. Freundlich equation.
Adsorption value (absolute BUT or excess G) in each case is determined by the temperature T and pressure R(with a gaseous adsorptive) or temperature T and concentration FROM(when adsorbed from solutions). As a rule, in the theory of adsorption, when considering the adsorption equilibrium, one of these parameters is kept constant. So, an equation of the form A \u003d f (p) T or G \u003d f (c) T, relating the amount of adsorption with pressure or concentration at a constant temperature, is called the adsorption isotherm. Adsorption (if it is expressed not as an excess, but as a total content) always increases with increasing equilibrium pressure or concentration. Since adsorption is an exothermic process, when the temperature rises, the value adsorption decreases. On fig. 26.9 shows the main types of adsorption equilibrium curves. Adsorption isotherms at three temperatures (T 1 > T 2 > T 3) corresponds to Fig. 26.9a.
Figure 26.9. Curves of adsorption equilibrium: isotherms (a), isobars (b) and isosteres (c) of adsorption
Equation relating adsorption value to temperature at constant equilibrium pressure A \u003d f (T) p or constant equilibrium concentration G \u003d f (T) s, is called, respectively, adsorption isobars or isopycnes (Fig. 26.9-b); here p 1 > p 2 > p 3. Type equation R= f (T) A, the adsorption isostere (Fig. 26.9-c), relates the equilibrium pressure to temperature at a constant adsorbed amount; in this case A 1 > A 2 > A 3.
The task of any adsorption theory is to compile its mathematical description on the basis of a certain model of the adsorption process. Ideally, the equation should describe the dependence of the equilibrium adsorption value on the concentration of the adsorbate in the bulk phase at different temperatures, and also predict the change in the heat of adsorption depending on the filling of the adsorbent. Most often, the adsorption isotherm equation is found in this case. The shape of the adsorption isotherm on solids depends on many parameters: the properties of the adsorbent and adsorbate, the interaction of the adsorbent adsorbate, the interaction of adsorbate molecules with each other in the gas phase and in the adsorbed state. In the range of low pressures (or concentrations) and the corresponding small surface coverages, the interaction between adsorbate molecules is insignificant and the dependence A = f(p) T reduced to its simplest form, called Henry's law:
A \u003d kp or A \u003d k "c(26.20)
where k And to"- adsorption coefficient (or Henry coefficient), from is the adsorbent concentration in the bulk phase, R is the vapor pressure of the adsorbate. Henry coefficient k is a measure of the intensity of adsorption. It can be shown that any theoretical isotherm should, in the limit (for small fillings), go over into the Henry equation.
In the region of medium concentrations, the concentration dependence of the adsorption of solutes is well described by the empirical Freundlich equation:
![]() (26.21)
(26.21)
where X- amount of adsorbed substance, m- adsorbent mass, βi P - constants characteristic of each adsorption system, with 0< 1/n< 1 . According to Freindlich, n does not depend on padding, although this statement is not entirely accurate. This empirical equation is often used for approximate calculations of adsorption. Most often it is used in logarithmic form:
which allows constructing a linear dependence ln BUT - ln c and graphically determine both constant parameters β and n.
Surface phenomena and adsorption. Types of adsorption interactions. Gas adsorption isotherms. Henry and Langmuir equation. Polymolecular adsorption, BET theory.
SURFACE PHENOMENA AND ADSORPTION
surface energy. Adsorption
Until now, the properties of heterogeneous systems have been described using parameters and state functions that characterize each of the phases as a whole. However, the properties of the phase area adjacent to its surface differ from the properties of the phase in the volume: in fact, the particles located on the surface of each phase form a special surface phase, the properties of which differ significantly from the properties of the internal regions of the phase. The particles located on the surface are in a different environment compared to the particles located in the volume of the phase, i.e. interact both with homogeneous particles and with particles of another kind. The consequence of this is that the average energy g s of a particle located on the phase interface differs from the average energy of the same particle in the volume of the phase g v (moreover, the energy of a particle on the surface can be either greater or less than the energy of a particle in the volume). Therefore, the most important characteristic of the surface phase is surface energy G s is the difference between the average energy of a particle located on the surface and a particle located in the volume of the phase, multiplied by the number of particles on the surface N:
![]() (26.1)
(26.1)
It is obvious that the total value of the surface energy of a phase will be determined by the value of its surface S. Therefore, to characterize the interface separating a given phase from another, the concept is introduced surface tensionσ is the ratio of the surface energy to the area of the interface; the magnitude of the surface tension depends only on the nature of both phases. Like the surface energy of a phase, surface tension can be either positive or negative. The surface tension is positive if the particles on the surface interact with particles of the same phase more strongly than with particles of another phase (and, therefore, g s > g v). According to the principle of minimum free energy, any phase will tend to spontaneously reduce its surface energy; therefore, in the case of positive surface tension (σ > 0), the phase tends to reduce its surface. If σ< 0, поверхностная энергия фазы будет уменьшаться при увеличении площади поверхности.
The influence of the surface layer of a phase on its general properties is determined by the proportion of particles located on the surface of the total number of particles that make up this phase, i.e. the value of the specific surface of the phase S/V (surface per unit volume). The free energy of the phase G can be represented as the sum of the surface G s and volume G v energies proportional to the surface area and volume of the phase, respectively:
Dividing this expression by the volume of the phase, we get:
It follows from equation (IV.4) that with the same amount of phase (i.e., constant volume), the contribution of the surface energy to the total energy of the phase increases with an increase in the specific surface area, or, in other words, degree of dispersion(fragmentation) phase. In the case when the degree of dispersion of the phase is small (the specific surface is insignificant), the contribution of the surface energy to the total energy of the phase is usually neglected. The contribution of the surface layer to the properties of the phase and the system as a whole is taken into account when studying dispersed systems– heterogeneous systems, one of the phases of which is continuous ( dispersion medium), and the other is fragmented ( dispersed phase).
At the boundary of a condensed (i.e., solid or liquid) phase with a gas, the surface tension is always positive, since the particles of the condensed phase interact with each other more strongly than with gas molecules. According to the principle of minimum free energy, the condensed phase will tend to spontaneously reduce its surface energy. This can be the result of either a decrease in the surface area of the phase (which is why a drop of liquid in weightlessness takes the form of a sphere), or a decrease in surface tension when new particles appear on the phase interface - gas molecules or a dissolved substance. The process of spontaneous change in the concentration of a substance at the interface between two phases is called adsorption. Adsorbent a substance is called, on the surface of which there is a change in the concentration of another substance - adsorbate.
Adsorption at the solution-vapor interface
In liquid solutions, surface tension σ is a function of the solute concentration. On fig. 4.1 shows three possible dependences of surface tension on the concentration of the solution (the so-called surface tension isotherms). Substances whose addition to a solvent reduces surface tension are called surface-active(surfactants), substances, the addition of which increases or does not change the surface tension - surface-inactive(PIAV).

Rice. 26.1 Surface isotherms 26.2 Adsorption isotherm
tension of surfactant solutions (1, 2) and PIAV. Surfactant at the solution-vapor interface
PIAV (3)
A decrease in surface tension and, consequently, surface energy occurs as a result of surfactant adsorption on the liquid-vapor interface, i.e. the fact that the concentration of surfactant in the surface layer of the solution is greater than in the depth of the solution.
The quantitative measure of adsorption at the solution-vapor interface is surface excess G (gamma), equal to the number of moles of solute in the surface layer. The quantitative relationship between the adsorption (surface excess) of a solute and the change in the surface tension of the solution with increasing solution concentration determines Gibbs adsorption isotherm:
The plot of the surfactant adsorption isotherm is shown in fig. 26.2. It follows from equation (26.5) that the direction of the process - the concentration of a substance in the surface layer or, conversely, its presence in the bulk of the liquid phase - is determined by the sign of the derivative dσ / dС. The negative value of this derivative corresponds to the accumulation of the substance in the surface layer (G > 0), the positive value corresponds to a lower concentration of the substance in the surface layer compared to its concentration in the bulk of the solution.
The value g = –dσ/dС is also called the surface activity of the solute. The surface activity of surfactants at a certain concentration of C 1 is determined graphically by drawing a tangent to the surface tension isotherm at the point C = C 1 ; in this case, the surface activity is numerically equal to the tangent of the slope of the tangent to the concentration axis:
It is easy to see that with increasing concentration, the surface activity of surfactants decreases. Therefore, the surface activity of a substance is usually determined at an infinitesimal concentration of the solution; in this case, its value, denoted g o, depends only on the nature of the surfactant and solvent. Investigating the surface tension of aqueous solutions of organic substances, Traube and Duclos established the following rule of thumb for the homologous series of surfactants:
In any homologous series at low concentrations, the elongation of the carbon chain by one CH2 group increases the surface activity by a factor of 3–3.5.
For aqueous solutions of fatty acids, the dependence of surface tension on concentration is described by the empirical Shishkovsky equation:
Here b and K are empirical constants, and the value of b is the same for the entire homological series, and the value of K increases for each subsequent member of the series by 3–3.5 times.

Rice. 26.3 Limit orientation of surfactant molecules in the surface layer
Molecules of most surfactants have a amphiphilic structure, i.e. contain both a polar group and a non-polar hydrocarbon radical. The location of such molecules in the surface layer is energetically most favorable under the condition that the molecules are oriented by the polar group to the polar phase (polar liquid), and the nonpolar group to the nonpolar phase (gas or nonpolar liquid). At a low concentration of the solution, thermal motion disrupts the orientation of surfactant molecules; with an increase in concentration, the adsorption layer is saturated and a layer of "vertically" oriented surfactant molecules is formed on the interface (Fig. 26.3). The formation of such a monomolecular layer corresponds to the minimum value of the surface tension of the surfactant solution and the maximum value of adsorption G (Fig. 26.1-26.2); with a further increase in the surfactant concentration in the solution, the surface tension and adsorption do not change.
Adsorption at the solid-gas interface
In the adsorption of gases on solids, the description of the interaction between adsorbate and adsorbent molecules is a very complex problem, since the nature of their interaction, which determines the nature of adsorption, can be different. Therefore, the problem is usually simplified by considering two extreme cases, when adsorption is caused by physical or chemical forces - respectively, physical and chemical adsorption.
physical adsorption arises due to van der Waals interactions. It is characterized by reversibility and a decrease in adsorption with increasing temperature, i.e. exothermicity, and the heat effect of physical adsorption is usually close to the heat of liquefaction of the adsorbate (10 – 80 kJ/mol). Such is, for example, the adsorption of inert gases on coal.
Chemical adsorption(chemisorption) is carried out by chemical interaction of adsorbent and adsorbate molecules. Chemisorption is usually irreversible; chemical adsorption, in contrast to physical adsorption, is localized; adsorbate molecules cannot move over the surface of the adsorbent. Since chemisorption is a chemical process that requires an activation energy of about 40-120 kJ/mol, an increase in temperature contributes to its occurrence. An example of chemical adsorption is the adsorption of oxygen on tungsten or silver at high temperatures.
It should be emphasized that the phenomena of physical and chemical adsorption are clearly distinguished in very rare cases. Intermediate options are usually carried out, when the bulk of the adsorbed substance binds relatively weakly and only a small part is firmly bound. For example, oxygen on metals or hydrogen on nickel at low temperatures are adsorbed according to the laws of physical adsorption, but as the temperature rises, chemical adsorption begins to occur. As the temperature rises, the increase in chemical adsorption from a certain temperature begins to overlap the drop in physical adsorption, so the temperature dependence of adsorption in this case has a clearly defined minimum (Fig. 26.4).

Rice. 26.4 Dependence of the volume of hydrogen adsorbed by nickel on temperature
At a constant temperature, the amount of adsorbed substance depends only on the equilibrium pressure or concentration of the adsorbate; the equation relating these quantities is called the adsorption isotherm.
Theories of adsorption
There is no unified theory that would adequately describe all types of adsorption on different phase interfaces; Therefore, let us consider some of the most common adsorption theories that describe individual types of adsorption on the solid-gas or solid-solution interface.
Langmuir's theory of monomolecular adsorption
The theory of monomolecular adsorption, which was developed by the American chemist I. Langmuir, is based on the following provisions.
1) Adsorption is localized and is caused by forces close to chemical ones.
2) Adsorption occurs not on the entire surface of the adsorbent, but on active centers, which are protrusions or depressions on the surface of the adsorbent, characterized by the presence of the so-called. free valencies. Active centers are considered independent (i.e. one active center does not affect the adsorption capacity of others), and identical.
3) Each active center is able to interact only with one adsorbate molecule; as a result, only one layer of adsorbed molecules can form on the surface.
4) The adsorption process is reversible and balanced– the adsorbed molecule is retained by the active center for some time, after which it is desorbed; thus, after some time, a dynamic equilibrium is established between the processes of adsorption and desorption.

Rice. 26.5 Monomolecular adsorption isotherm
In the state of equilibrium, the rate of adsorption is equal to the rate of desorption. The desorption rate is directly proportional to the proportion of occupied active centers (x), and the adsorption rate is directly proportional to the product of the adsorbate concentration and the fraction of free active centers (1 – x):
![]() (26.9)
(26.9)
From here we find x:
Dividing the numerator and denominator of the right side of equation (26.10) by k A , we get:
 (26.11)
(26.11)
The maximum possible value of adsorption T o is achieved under the condition that all active centers are occupied by adsorbate molecules, i.e. x = 1. Hence it follows that x = r / r o. Substituting this into equation (26.11), we get:
Equation (26.13) is monomolecular adsorption isotherm, which relates the value of adsorption G to the concentration of adsorbate C. Here b is some constant value for a given adsorbent-adsorbate pair (the ratio of desorption and adsorption rate constants), numerically equal to the adsorbate concentration, at which half of the active centers are occupied. Schedule Langmuir adsorption isotherms shown in fig. 26.5. The constant b can be determined graphically by drawing a tangent to the adsorption isotherm at the point C = 0.
When describing the process of adsorption of gases in equation (26.13), the concentration can be replaced by a proportional value of the partial pressure of the gas:
Langmuir's theory of monomolecular adsorption is applicable to describe some processes of adsorption of gases and dissolved substances at low pressures (concentrations) of the adsorbate.
Polanyi's theory of polymolecular adsorption
In practice, often (especially in the adsorption of vapors) there are so-called. S-shaped adsorption isotherms (Fig. 4.6), the shape of which indicates the possible, starting from a certain pressure value, the interaction of adsorbed molecules with the adsorbate.

Rice. 26.6 Polymolecular adsorption isotherm
To describe such adsorption isotherms, M. Polyani proposed theory of polymolecular adsorption based on the following main principles:
1. Adsorption caused purely physical forces.
2. Adsorbent surface homogeneous, i.e. there are no active centers on it; adsorption forces form a continuous force field near the surface of the adsorbent.
3. Adsorption forces act at a distance greater than the size of the adsorbate molecule. In other words, at the surface of the adsorbent there is some adsorption volume, which is filled with adsorbate molecules during adsorption.
4. The attraction of an adsorbate molecule by the adsorbent surface does not depend on the presence of other molecules in the adsorption volume, as a result of which it is possible polymolecular adsorption.
5. Adsorption forces do not depend on temperature and, consequently, with a change in temperature, the adsorption volume does not change.
Freundlich equation
The theoretical concepts developed by Langmuir and Polanyi largely idealize and simplify the true picture of adsorption. In fact, the surface of the adsorbent is inhomogeneous, there is an interaction between the adsorbed particles, active centers are not completely independent of each other, etc. All this complicates the form of the isotherm equation. G. Freindlich showed that at a constant temperature, the number of moles of adsorbed gas or solute per unit mass of the adsorbent (the so-called specific adsorption x / m) is proportional to the equilibrium pressure (for gas) or equilibrium concentration (for substances adsorbed from solution) of the adsorbent raised to a certain power, which is always less than one:
Adsorption at the solid-solution interface
Molecular adsorption from solutions
Adsorption isotherms of dissolved substances from a solution are similar in appearance to adsorption isotherms for gases; for dilute solutions, these isotherms are well described by the Freundlich or Langmuir equations, if the equilibrium concentration of the solute in the solution is substituted into them. However, adsorption from solutions is a much more complex phenomenon compared to gaseous one, since the adsorption of a solvent often occurs simultaneously with the adsorption of a solute.

Rice. 26.8 Orientation of surfactant molecules on the adsorbent surface
The dependence of adsorption on the structure of adsorbate molecules is very complex, and it is rather difficult to derive any regularities. Molecules of many organic substances consist of polar (hydrophilic) and non-polar (hydrophobic) groups, i.e. are surfactants. When adsorbed on a solid adsorbent, surfactant molecules are oriented on its surface in such a way that the polar part of the molecule faces the polar phase, and the nonpolar part faces the nonpolar phase. So, during the adsorption of aliphatic carboxylic acids from aqueous solutions on a nonpolar adsorbent - activated carbon - the molecules are oriented by hydrocarbon radicals towards the adsorbent; when adsorbed from benzene (non-polar solvent) on a polar adsorbent - silica gel - the orientation of the acid molecules will be reversed (Fig. 4.8).
Adsorption from electrolyte solutions
Adsorption from aqueous solutions of electrolytes occurs, as a rule, in such a way that ions of the same type are adsorbed from the solution on the solid adsorbent. Preferential adsorption from a solution or an anion or a cation is determined by the nature of the adsorbent and ions. The mechanism of adsorption of ions from electrolyte solutions can be different; allocate exchange and specific adsorption of ions.
Exchange adsorption is a process of ion exchange between a solution and a solid phase, in which the solid phase absorbs ions of a certain sign (cations or anions) from the solution and instead releases an equivalent number of other ions of the same sign into the solution. Exchange adsorption is always specific, i.e. for a given adsorbent, only certain ions are capable of exchange; exchange adsorption is usually irreversible.
At specific adsorption adsorption on the surface of the solid phase of ions of any kind is not accompanied by the release into the solution of an equivalent number of other ions of the same sign; the solid phase acquires an electric charge. This leads to the fact that near the surface, under the action of electrostatic attraction forces, an equivalent number of ions with opposite charges are grouped, i.e. an electrical double layer is formed. The interaction of charges concentrating on the surface leads to a decrease in the surface energy of the system. For the case of specific electrolyte adsorption, Peskov and Fayans formulated the following empirical rule ( Peskov-Faience rule):
On the surface of a crystalline solid, an ion is specifically adsorbed from an electrolyte solution, which is able to complete its crystal lattice or can form a poorly soluble compound with one of the ions that make up the crystal.
Henry's law can be formulated as follows: when the system is diluted (pressure decreases), the distribution coefficient tends to a constant value equal to the Henry distribution constant. With respect to the adsorption value A, this law can be written as follows:
E  These equations are adsorption isotherms of a substance at low concentrations. In accordance with them, Henry's law can be formulated as follows: the amount of adsorption at low gas pressures (substance concentrations in solution) is directly proportional to pressure (concentration).
These equations are adsorption isotherms of a substance at low concentrations. In accordance with them, Henry's law can be formulated as follows: the amount of adsorption at low gas pressures (substance concentrations in solution) is directly proportional to pressure (concentration).
Deviations from Henry's law, expressed by changes in the activity coefficients in the phases, usually do not allow one to describe and predict the course of isotherms with increasing concentration.
(pressure) of the adsorbate. To obtain a theoretical adsorption isotherm describing a wider range of concentrations, it is necessary to use concepts of the adsorption mechanism and specific models.
A large fraction of deviations of the activity coefficient of the adsorbate in the surface layer from unity can be taken into account using the concept of adsorption as a quasi-chemical reaction between the adsorbate and the adsorption centers of the adsorbent surface. This is the main idea of Langmuir's adsorption theory. This provision is clarified by the following assumptions:
1) adsorption is localized (molecules do not move over the surface) on separate adsorption centers, each of which interacts with only one adsorbate molecule; as a result, a monomolecular layer is formed;
2) adsorption centers are energetically equivalent - the surface of the adsorbent is equipotential;
3) adsorbed molecules do not interact with each other.
Lyophilic disperse systems. Classification and general characteristics of pav. Thermodynamics and mechanism of micellization. The structure of surfactant micelles in aqueous and hydrocarbon media. Solubilization.
All dispersed systems, depending on the mechanism of their formation, according to the classification of P. A. Rebinder, are divided into lyophilic, which are obtained by spontaneous dispersion of one of the phases (spontaneous formation of a heterogeneous free-dispersed system), and lyophobic, resulting from dispersion and condensation with supersaturation (forced formation of a heterogeneous free-range system).
If, with increasing concentration of a substance, the surface tension at the interface decreases, then such a substance is called surface-active. For such substances, the surface activity
The presence of hydrophilic and oleophilic parts in surfactant molecules is a characteristic distinguishing feature of their structure. According to the ability to dissociate in aqueous solutions, surfactants are divided into ionic and nonionic. In turn, ionic surfactants are divided into anionic, cationic and ampholytic (amphoteric).
1) Anionic surfactants dissociate in water to form a surface-active anion.
2) Cationic surfactants dissociate in water to form a surface-active cation.
3) Ampholytic surfactants contain two functional groups, one of which is acidic and the other basic, such as carboxyl and amine groups. Depending on the pH of the medium, ampholytic surfactants exhibit anionic or cationic properties.
All surfactants with respect to their behavior in water are divided into truly soluble and colloidal.
Truly soluble surfactants in solution are in a molecularly dispersed state up to concentrations corresponding to their saturated solutions and the separation of the system into two continuous phases.
The main distinguishing feature of colloidal surfactants is the ability to form thermodynamically stable (lyophilic) heterogeneous disperse systems (associative, or micellar, colloids). The main properties of colloidal surfactants, which determine their valuable qualities and wide application, include high surface activity; the ability to spontaneous micelle formation - the formation of lyophilic colloidal solutions at a surfactant concentration above a certain specific value, called the critical micelle concentration (KKM); the ability to solubilize - a sharp increase in the solubility of substances in solutions of colloidal surfactants due to their "introduction" into the micelles; high ability to stabilize various disperse systems.
At concentrations above KKM, surfactant molecules are collected into micelles (associate) and the solution transforms into a micellar (associative) colloidal system.
A surfactant micelle is understood as an associate of amphiphilic molecules, the lyophilic groups of which are facing the corresponding solvent, and the lyophobic groups are connected to each other, forming the core of the micelle. The number of molecules that make up a micelle is called the association number, and the total sum of the molecular weights of the molecules in the micelle, or the product of the mass of the micelle and the Avogadro number, is called the micellar mass. A certain orientation of amphiphilic surfactant molecules in a micelle provides a minimum interfacial tension at the micelle-environment boundary.
P  At concentrations of surfactants in an aqueous solution that are somewhat higher than KKM, according to Hartley's concepts, spherical micelles (Hartley micelles) are formed. The inner part of Gartley micelles consists of intertwining hydrocarbon radicals, the polar groups of surfactant molecules are turned into the aqueous phase. The diameter of such micelles is equal to twice the length of surfactant molecules. The number of molecules in a micelle grows rapidly within a narrow concentration range, and with a further increase in concentration, it practically does not change, but the number of micelles increases. Spherical micelles can contain from 20 to 100 molecules or more.
At concentrations of surfactants in an aqueous solution that are somewhat higher than KKM, according to Hartley's concepts, spherical micelles (Hartley micelles) are formed. The inner part of Gartley micelles consists of intertwining hydrocarbon radicals, the polar groups of surfactant molecules are turned into the aqueous phase. The diameter of such micelles is equal to twice the length of surfactant molecules. The number of molecules in a micelle grows rapidly within a narrow concentration range, and with a further increase in concentration, it practically does not change, but the number of micelles increases. Spherical micelles can contain from 20 to 100 molecules or more.
As the surfactant concentration increases, the micellar system passes through a series of equilibrium states that differ in association numbers, sizes, and shapes of micelles. When a certain concentration is reached, spherical micelles begin to interact with each other, which contributes to their deformation. Micelles tend to take a cylindrical, disc-shaped, rod-shaped, lamellar shape.
Micellization in non-aqueous media, as a rule, is the result of the action of attractive forces between the polar groups of surfactants and the interaction of hydrocarbon radicals with solvent molecules. The inverted micelles formed contain non-hydrated or hydrated polar groups inside, surrounded by a layer of hydrocarbon radicals. The association number (from 3 to 40) is much less than for aqueous solutions of surfactants. As a rule, it grows with an increase in the hydrocarbon radical up to a certain limit.
The phenomenon of dissolution of substances in surfactant micelles is called solubilization. The way in which solubilizate molecules are included in micelles in aqueous solutions depends on the nature of the substance. Non-polar hydrocarbons, penetrating into micelles, are located in the hydrocarbon cores of micelles. Polar organic substances (alcohols, amines, acids) are incorporated into a micelle between surfactant molecules so that their polar groups face water, and the lipophilic parts of the molecules are oriented parallel to the surfactant hydrocarbon radicals. A third way of incorporating the solubilizate into micelles is also possible, which is especially characteristic of nonionic surfactants. Solubilizate molecules, such as phenol, do not penetrate into micelles, but are fixed on their surface, located between randomly bent polyoxyethylene chains.
Solubilization is a spontaneous and reversible process; a given surfactant concentration and temperature corresponds to a well-defined saturation of the solution with solubilizate. As a result of solubilization, stable disperse systems are obtained similar to spontaneously formed ultramicroheterogeneous emulsions.
Determine the surface and total (internal) energy of 4 g of water mist, which has particles with a dispersion of 5 10 7 m -1 , t= 20ºC, σ = 72 mJ/m 2 ; dσ/ dT= - 0.16 mJ/(m 2 ·TO); ρ = 1000 kg/m 3 .

Examination ticket number 10
Theory of polymolecular BET adsorption: initial positions, derivation of the isotherm equation and its analysis. Linear form of the BET equation. Determination of the specific surface of adsorbents, catalysts and other porous bodies.
The Langmuir equation can be used only under the condition that the adsorption of a substance is accompanied by the formation of a monomolecular layer.
In most cases, the monomolecular adsorption layer does not fully compensate for the excess surface energy, and the influence of surface forces can extend to the second, third, and subsequent adsorption layers, resulting in polymolecular adsorption.
FROM  The modern form of the polymolecular adsorption equation - the basic equation of the generalized Langmuir theory - was proposed by Brunauer, Emmett and Teller.
The modern form of the polymolecular adsorption equation - the basic equation of the generalized Langmuir theory - was proposed by Brunauer, Emmett and Teller.
In this theory, an additional assumption to those that were used as the basis for the derivation of the Langmuir isotherm equation is the idea of the formation on the surface of the adsorbent of "successive complexes" of adsorption centers with one, two, three, etc. molecules of the adsorbate. Then the adsorption process can be represented as successive quasi-chemical reactions:
The equilibrium constants of these reactions are respectively equal to
Denote:
The total number of active sites on the adsorbent, or the capacity of the monolayer, will be equal to

After a series of calculations using the theory of series, we finally get:

D  This relation is the basic equation of the generalized Langmuir theory and is called the BET polymolecular adsorption equation.
This relation is the basic equation of the generalized Langmuir theory and is called the BET polymolecular adsorption equation.
When processing experimental results, the BET equation is usually used in a linear form:
It allows you to graphically determine both constant parameters A ∞ and С:
The experimental determination of A ∞ makes it possible to calculate the specific surface area of the adsorbent (surface area per unit mass of the adsorbent): ![]() .
.
Adsorption- concentration of a substance from the volume of phases on the interface between them. Adsorption can be seen as absorption substance (adsorbate) surface of the adsorbent.
Adsorbent The substance on whose surface adsorption takes place.
Adsorbtiv - a gas or solute capable of being adsorbed on the surface of an adsorbent.
Adsorbate - adsorbed substance on the surface of the adsorbent. Often the concepts of "adsorbent" "adsorbate" are identified
Distinguish physical adsorption, occurring without chemical change of the adsorbate and chemical adsorption(chemisorption), accompanied by chemical interaction of the adsorbent with the adsorbent.
Adsorption happens at the phase boundaries: solid - liquid, solid - gas, liquid - gas, liquid - liquid.
When a substance is adsorbed in the form of molecules, it is called molecular adsorption, in the form of ions - ionic adsorption.
Adsorption is reversible, the reverse process is called desorption.
The rates of adsorption and desorption are equal to each other at adsorption equilibrium, which corresponds to equilibrium concentration adsorbate in solution or equilibrium pressure in the gas phase.
Adsorption value(A) is characterized by the equilibrium amount of absorbed substance (X) per unit mass of solid adsorbent (m): [mol/kg or kg/kg]
Adsorption isotherm- graphical representation of the dependence of the adsorption value on the equilibrium concentration or equilibrium pressure at a given constant temperature.
Distinguish adsorption monomolecular, at which the adsorbate covers the surface of the adsorbent with a layer one molecule thick and polymolecular, at which the adsorbate molecules can be located on the surface of the adsorbent in several layers.
Monomolecular adsorption isotherm has the form shown in Fig. 12 ( Langmuir isotherm)
A Site I - answers small equilibrium concentrations (pressures), when a small part of the adsorbent surface is occupied by adsorbate molecules, and the dependence A - c (p) is linear;
Section II - medium concentrations (pressures) at which a significant proportion of the adsorbent surface is occupied by adsorbate molecules;
c (p) Section III - observed at high equilibrium concentrations (pressures), when the entire adsorbent surface is occupied by adsorbate molecules and reached limit value of adsorption (A).
Isotherm of monomolecular adsorption well is described by the Langmuir equation:

where c, A constants individual for each individual substance during adsorption on a specific adsorbent;
s, r- equilibrium concentration or equilibrium pressure.
At low equilibrium concentrations, we can neglect the value from or R in the denominator. Then the Langmuir equation is transformed into the equation of a straight line passing through the origin:
A = A in c or A \u003d A in p
At high equilibrium concentrations can be neglected in the denominator in. Then the Langmuir equation is transformed into the equation of a straight line independent of from or R: A = A
For practical calculations it is necessary to know the constants of the Langmuir equation A and in. Transforming the equation into a linear form of a straight line that does not pass through the origin of coordinates: , allows you to build a graph of the dependence 1/A - 1/c (Fig. 13).
1/A The segment OB is equal to 1/A. Coefficient in can be found from the fact that in is equal to the concentration at which the amount of adsorption is half of the limit.

On the graph, interpolation determines the segment OD corresponding to 2/A and equal to 1/in. Then s = 1/OD.
The Langmuir equation was derived from the theory of monomolecular adsorption, which has the following basic provisions:
adsorption of molecules occurs only on adsorption centers (tops of irregularities and narrow pores);
each adsorption center can hold only one adsorbate molecule;
the adsorption process is reversible; adsorption equilibrium is dynamic. Adsorbed molecules are retained by adsorption centers only for a certain time, after which these molecules are desorbed and the same number of new molecules are adsorbed.
In addition to the Langmuir equation, in practice it is often used Freundlich equation:
A \u003d KS 1 / n or A \u003d KR 1 / n, where K and 1 / n are empirical constants.
The equation is more suitable to describe adsorption on the porous or powdered adsorbents in the area average concentrations (pressures).
The Freundlich adsorption isotherm does not have a horizontal straight line and adsorption increases with increasing concentration (pressure) (Fig. 14).

Rice. fourteen
For finding the constants of the Freundlich equation it is converted using the logarithm into the equation of a straight line that does not pass through the origin: lg A \u003d log K + 1 / n lg C.
In accordance with this, the graph of the dependence of lg A on lg C or (P), built according to experimental data, has the form shown in Fig. 15. By extrapolation to the ordinate axis, a segment OB is obtained equal to lg K. The tangent of the angle of inclination of the straight line BN to the abscissa axis is 1/n ( tg =)
Polymolecular adsorption- observed during adsorption on porous or powdered adsorbents (silica gel, activated carbon, powders and tablets of medicinal substances). In this case, adsorption continues until a dense monomolecular layer is formed, as shown in Fig. 16.

Rice. 16.

Such adsorption corresponds to another type of isotherm (Fig. 17), the so-called " S - isotherm".
capillary condensation- the phenomenon of vapor liquefaction in the pores or capillaries of a solid adsorbent, it is observed when easily liquefied gases or vapors (for example, water, benzene, etc.) are absorbed as a result of polymolecular adsorption. Wherein polymolecular layer represents thin film of liquid covering the inner surface of the pore. Layers of such a liquid merging with each other in narrow places form concave menisci, under which vapor pressure is created. Thereby pores draw in gas molecules (vapour) and filled with liquid formed during condensation.
When flowing adsorption complicated by capillary condensation, the isotherm corresponding to the filling of pores (1) does not coincide with the isotherm (2) corresponding to their emptying (Fig. 18). On the isotherm, condensation hysteresis loop. The processes of adsorption and desorption do not coincide.





 - sphere,
- sphere, ,
,


